Clinical Trials Market, (By Phase Type: Phase I, Phase II, Phase III, Phase IV & Others .By Design Type: Interventional, Observational, Expanded Access & Others. By Region - North America, Europe , Asia-Pacific , LAMEA) - Global Market Analysis, Growth, Trends & Forecast, 2022 to 2032
Report Type : Syndicate Report
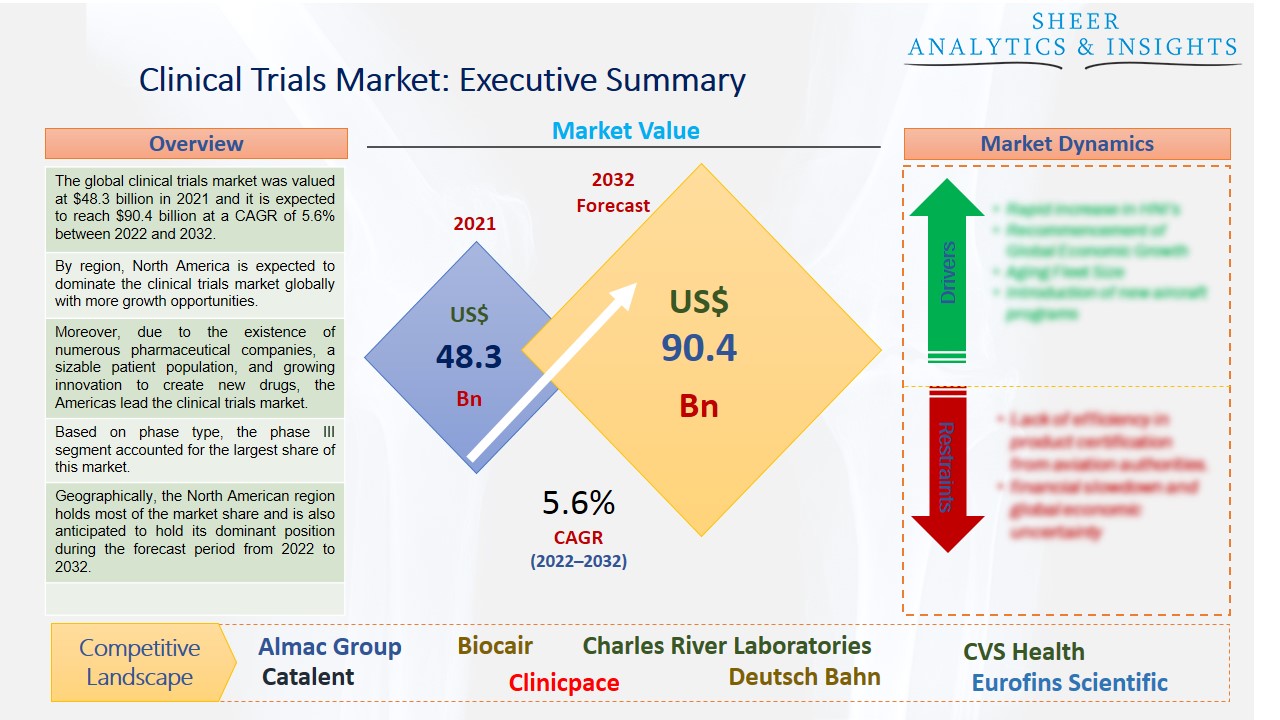
The global clinical trials market was valued at $48.3 billion in 2021 and it is expected to reach $90.4 billion at a CAGR of 5.6% between 2022 and 2032. The market will grow as a result of therapeutic approaches including personalized medicine, cutting-edge technology, and the rising demand for contract research organizations to carry out clinical studies. The rise of the market will be aided by the rising prevalence of chronic diseases, a rise in the need for biologists, and a rise in the amount of money invested in pharmaceutical industry R&D.
By region, North America is expected to dominate the clinical trials market globally with more growth opportunities.
Clinical trials are research projects conducted on human subjects to assess a therapeutic, surgical, or behavioral intervention. They are the main method by which scientists determine whether a novel therapy, such as a new medicine, food, or medical equipment, is secure and efficient in humans. A clinical trial is frequently performed to determine whether a new treatment is more efficient than the current treatment and/or has fewer negative side effects. In addition, other clinical studies examine methods for detecting diseases early on, often even before symptoms appear. Others research strategies to avoid health issues. A clinical trial may also focus on ways to improve the quality of life for those who have chronic health issues or life-threatening illnesses.
Source: SAI Research
Download Free PDF Sample Request
To manage capacity and access scientific and process advancements to generate cost-effective and efficient drug molecules, it has become important to outsource various drug development phases due to the growing number of medications in the pipeline. The market for clinical trials is anticipated to develop as a result of this trend. To increase profitability, adhere to the timescales required for drug development, and save expenses, the majority of pharmaceutical and biopharmaceutical businesses often outsource their testing functions during R&D. The most recent contracts between big pharmacies and CROs are proof of this.
Moreover, due to the existence of numerous pharmaceutical companies, a sizable patient population, and growing innovation to create new drugs, the Americas lead the clinical trials market. Additionally, the growing numbers of patients with cancer, diabetes, and other life-threatening illnesses encourage the use of clinical trial techniques to create new treatment choices. The National Cancer Institute participates in clinical studies on many cancer kinds to create cures. Furthermore, the National Cancer Institute participates in clinical studies on many cancer kinds to create cures. The second-largest market in Europe is for clinical trials. It is expected that factors including the pharmaceutical industries strong R&D spending, rising disease prevalence and an increase in new disease cases will further boost the clinical trials market. To strongly encourage scientific research and industrial advancement, the European Commission has adopted new laws in clinical trials in Europe.
Based on phase type, the phase III segment accounted for the largest share of this market. This is so because Phase III trials cost the most money and have the largest subject populations. Phase III clinical trials confirm and further explore the safety and efficacy findings from Phase 1 and 2 trials by comparing the new medication's effects to those of existing or previously studied drugs. Due to their increasing complexity and requirement for a bigger patient pool, phase III clinical studies continue to be more expensive than phase I and phase II trials. Consequently, this market sector is anticipated to experience more growth potential in the future.
In terms of design type, the interventional study dominated the market over the past few years. The majority of all investigations are interventional studies, with the clinical procedure, behavioral, and device intervention research coming in second and third. Interventional studies make up the biggest portion of all investigations. The demand for clinical trials has increased tremendously as a result of the need for clinical trials in the development of diagnostic tools and vaccinations for viral infections. Therefore, a significant factor in the large revenue share of interventional studies is the high prevalence of emerging viral diseases and ongoing technological advancements in clinical trials.
Geographically, the North American region holds most of the market share and is also anticipated to hold its dominant position during the forecast period from 2022 to 2032. The pharmaceutical industry's focus on developing new drugs to treat a variety of chronic conditions is predicted to increase industry income. Due to its economic vitality, this region frequently enjoys the first-mover advantage in practically every technology disruption. In the case of clinical trials, pharmaceutical companies are employing artificial intelligence to disrupt the entire process. This might be ascribed to a growth in new technology use in clinical trials in this area as well as increased R&D investments. For instance, industry leaders like IQVIA and PRA Health Sciences expect to further drive the growth of the North American market by implementing virtual services in various stages of clinical trials. On the other hand, the Asia-Pacific region is also expected to become the second-largest market in the future. Due to the presence of a sizable patient pool that facilitates easy recruitment, the Asia-Pacific area is also anticipated to overtake the United States as the second-largest market.
According to the study, key players such as Almac Group (U.K), Biocair (U.K), Charles River Laboratories (U.S), CVS Health (U.S), Catalent (U.S), Clinicpace (U.S), Deutsch Bahn (Germany), Eurofins Scientific (France), Eli Lilly and Company (U.S), IQVIA (U.S), ICON Plc (Ireland), Kendle (U.S), LabCorp (U.S), Novo Nordisk (Denmark), Pfizer (U.S), Medpace (U.S), Parexel (U.S), SGS SA (Switzerland), Syneos Health (U.S), Thermo Fisher Scientific (U.S), Wuxi AppTec (China), among others are leading the global clinical trials market.
Scope of the Report:
| Report Coverage | Details |
| Market Size in 2021 | US$ 48.3 Billion |
| Market Volume Projection by 2032 | US$ 90.4 Billion |
| Forecast Period 2022 to 2032 CAGR | 5.6% |
| Base Year: | 2021 |
| Historical Data | 2019, 2020 and 2021 |
| Forecast Period | 2022 to 2032 |
| Segments covered |
By Phase Type: Phase I, Phase II, Phase III, Phase IV & Others By Design Type: Interventional, Observational, Expanded Access & Others |
| Geographies covered |
North America, Europe, Asia-Pacific, LAMEA |
| Companies covered | Almac Group (U.K), Biocair (U.K), Charles River Laboratories (U.S), CVS Health (U.S), Catalent (U.S), Clinicpace (U.S), Deutsch Bahn (Germany), Eurofins Scientific (France), Eli Lilly and Company (U.S), IQVIA (U.S), ICON Plc (Ireland), Kendle (U.S), LabCorp (U.S), Novo Nordisk (Denmark), Pfizer (U.S), Medpace (U.S), Parexel (U.S), SGS SA (Switzerland), Syneos Health (U.S), Thermo Fisher Scientific (U.S), Wuxi AppTec (China), among others. |
The Global Clinical Trials Market Has Been Segmented Into:
The Global Clinical Trials Market – by Phase Type:
- Phase I
- Phase II
- Phase III
- Phase IV
- Others
The Global Clinical Trials Market – by Design Type:
- Interventional
- Observational
- Expanded Access
- Others
The Global Clinical Trials Market – by Regions:
- North America
- The U.S.
- Canada
- Mexico
- Europe
- U.K.
- France
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- India
- China
- Japan
- Australia
- Rest of Asia Pacific
- LAMEA
- Middle East
- Saudi Arabia
- UAE
- Others
- Latin America
- Brazil
- Chile
- Others
- Africa
- South Africa
- Egypt
- Others
Buy Chapters or Sections
Customization options available to meet your custom research requirements :
- Request a part of this report
- Get geography specific report
- Request historical analysis
- Check out special discounted pricing

