Asia-Pacific Clinical Trials Market, (By Phase Type: Phase I, Phase II, Phase III, Phase IV & Others. By Indication: Pain Management, Oncology, CNS Condition, Diabetes, Obesity, Cardiovascular & Others. By Region - Asia-Pacific) -Industry Market Analysis, Growth, Trends & Forecast, 2022 to 2032
Report Type : Syndicate Report
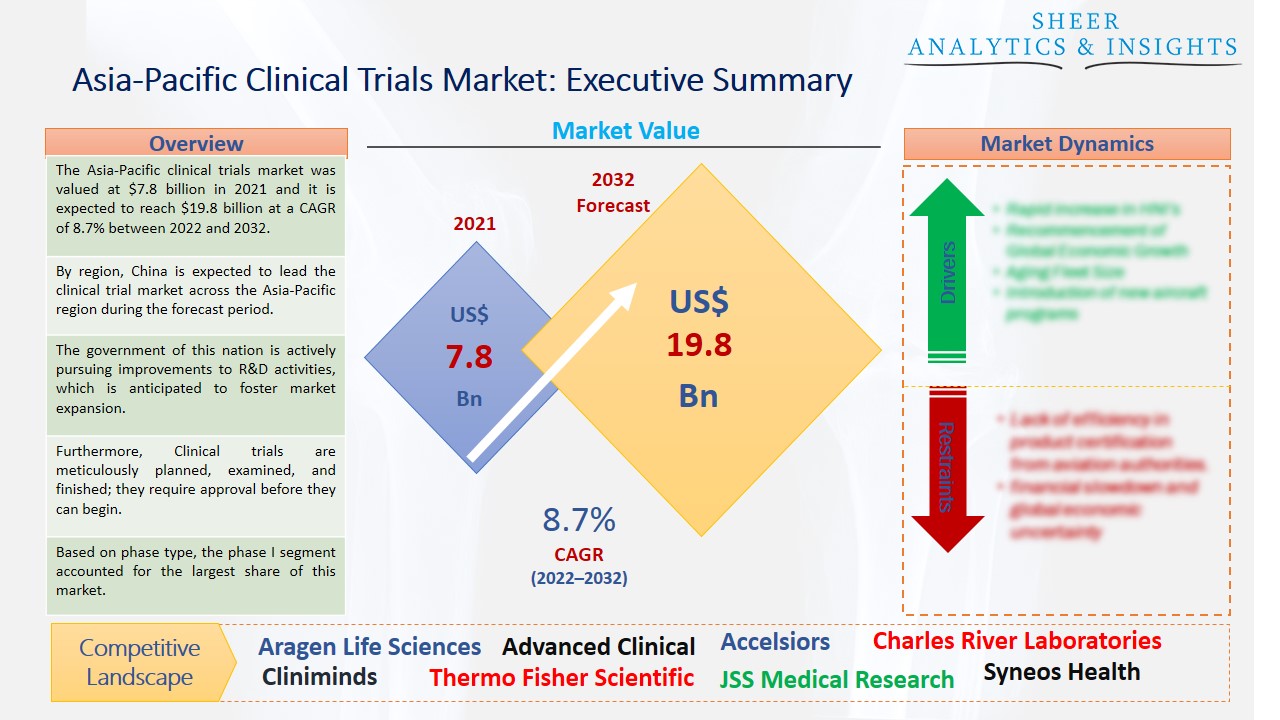
The Asia-Pacific clinical trials market was valued at $7.8 billion in 2021 and it is expected to reach $19.8 billion at a CAGR of 8.7% between 2022 and 2032. The region's healthcare IT industry is being upgraded, which is expected to result in constant growth for the Asia Pacific clinical trials market. One significant development that affects the significance of the sector is the widespread adoption of digital solutions across the domain. The collecting and storage of medical records are made significantly simpler when these solutions are used, assisting physicians with effective workflow.
By region, China is expected to lead the clinical trial market across the Asia-Pacific region during the forecast period.
A form of research called clinical trials examines novel diagnostic and therapeutic approaches and assesses how they affect patient outcomes. Clinical trials involving the testing of medications, cells, and other biological products, surgical techniques, radiological techniques, gadgets, behavioral treatments, and preventative care are conducted with the help of volunteers. A treatment's cost-effectiveness, the clinical utility of a diagnostic test, and how treatment affects the quality of life are all key topics covered in clinical trials. In addition, Phases of clinical trials are carried out. Each stage aims to provide answers to specific queries while taking the necessary precautions to protect the participants. Before regulatory organizations deem a new medication safe and effective, it is often put through three phases of clinical trials.
Source: SAI Research
Download Free PDF Sample Request
The government of this nation is actively pursuing improvements to R&D activities, which is anticipated to foster market expansion. Additionally, the digitalization of clinical trials is anticipated to benefit market expansion. Several trial operations, including data collecting, regulatory compliance, logistics, and supply management, among others, have been made more efficient thanks to digitalization. Additionally, the advent of digital therapies has made it simpler than ever to collect real-time data on safety and toxicity, supporting quick trial design correction and fostering market expansion.
Furthermore, Clinical trials are meticulously planned, examined, and finished; they require approval before they can begin. Clinical trials are open to participants of various ages, even young toddlers. The need for new medical equipment and pharmaceuticals among end users, together with rising investments in research and development operations for the creation of efficient medications, are the key drivers of the worldwide clinical trials market.
Based on phase type, the phase I segment accounted for the largest share of this market and is anticipated to accelerate the market growth over the forecast period. The initial step in a clinical trial is called a phase I study, which looks at how and where medicine or vaccine is dispersed in the body and makes sure it is safe for humans. The country's demand for novel medications and biologics is also boosting the segment's expansion. On the other hand, the phase III category is also expected to lead the market with more growth opportunities in the future. This is explained by the segment's high-cost structure. One of the main factors contributing to this trial's high price is the requirement for a large patient group in phase III trials.
In terms of indication type, the oncology segment accounted for the largest share of the Asia-Pacific clinical trials market. This is due to the rising incidence of cancer in the nation and the rising demand for cutting-edge medical technology and cancer therapies. By 2040, it is anticipated that 2 million individuals in India would have cancer, which is one of the leading causes of death in the world. For instance, liver cancer occurs at a significant rate. South Korea requires that there be more clinical trials conducted there in connection with research on cancer treatments.
Based on regions, China has most of the market share. Additionally, most of the key players are located in several places in this country which is another advantage for the clinical trials market. In addition to clinical research journals, scientific conferences, and other sources, China sources clinical trials through a variety of international government, non-government, and commercial clinical trial registries. Moreover, some of the key market growth drivers at the national level include the emergence of serious diseases, the sizeable patient population, an increase in clinical trials, and R&D. The regional government also provides financing for drug development research and related investigations.
According to the study, key players such as Aragen Life Sciences (India), Advanced Clinical (India), Alcura (U.K), Accelsiors (Hungary), Charles River Laboratories (U.S), Cliniminds (India), ERT (U.S), IQVIA (U.S), ICON PLC (India), JSS Medical Research (Canada), J.K Organization (India), LabCorp (U.S), PSI CRO (Switzerland), Parexel (U.S), Syneos Health (U.S), SGS SA (Switzerland), Thermo Fisher Scientific (U.S), Veeva Systems (U.S), Wuxi Apptec (China), Wipro (India), among others are leading the Asia-Pacific clinical trials market.
Scope of the Report:
| Report Coverage | Details |
| Market Size in 2021 | US$ 7.8 Billion |
| Market Volume Projection by 2032 | US$ 19.8 Billion |
| Forecast Period 2022 to 2032 CAGR | 8.7% |
| Base Year: | 2021 |
| Historical Data | 2019, 2020 and 2021 |
| Forecast Period | 2022 to 2032 |
| Segments covered |
By Phase Type: Phase I, Phase II, Phase III, Phase IV & Others By Indication Type: Pain Management, Oncology, CNS Condition, Diabetes, Obesity, Cardiovascular & Others |
| Geographies covered |
Asia Pacific: India, China, Japan, South Korea, Australia, Singapore, Thailand, Sri Lanka & Others |
| Companies covered | Aragen Life Sciences (India), Advanced Clinical (India), Alcura (U.K), Accelsiors (Hungary), Charles River Laboratories (U.S), Cliniminds (India), ERT (U.S), IQVIA (U.S), ICON PLC (India), JSS Medical Research (Canada), J.K Organization (India), LabCorp (U.S), PSI CRO (Switzerland), Parexel (U.S), Syneos Health (U.S), SGS SA (Switzerland), Thermo Fisher Scientific (U.S), Veeva Systems (U.S), Wuxi Apptec (China), Wipro (India), among others. |
The Asia-Pacific Clinical Trials Market Has Been Segmented Into:
The Asia-Pacific Clinical Trials Market – by Phase Type:
- Phase I
- Phase II
- Phase III
- Phase IV
- Others
The Asia-Pacific Clinical Trials Market – by Indication Type:
- Pain Management
- Oncology
- CNS Condition
- Diabetes
- Obesity
- Cardiovascular
- Others
The Asia-Pacific Clinical Trials Market – by Regions
Asia Pacific
- India
- China
- Japan
- South Korea
- Australia
- Singapore
- Thailand
- Sri Lanka
- Others
Buy Chapters or Sections
Customization options available to meet your custom research requirements :
- Request a part of this report
- Get geography specific report
- Request historical analysis
- Check out special discounted pricing

