Clinical Trial Management System Market (By Solution Type: Site, Enterprise. By Component Type: Software, Services. By Delivery Mode Type: Web-based CTMS, On-Premise, Cloud-based. By End-User Type: Large Pharma Bio-tech Companies, Small and Mid-sized Pharma Bio-tech Companies, CROs, Others. By Region - North America, Europe , Asia-Pacific , LAMEA) - Global Market Analysis, Growth, Trends & Forecast, 2022-2032
Report Type : Syndicate Report
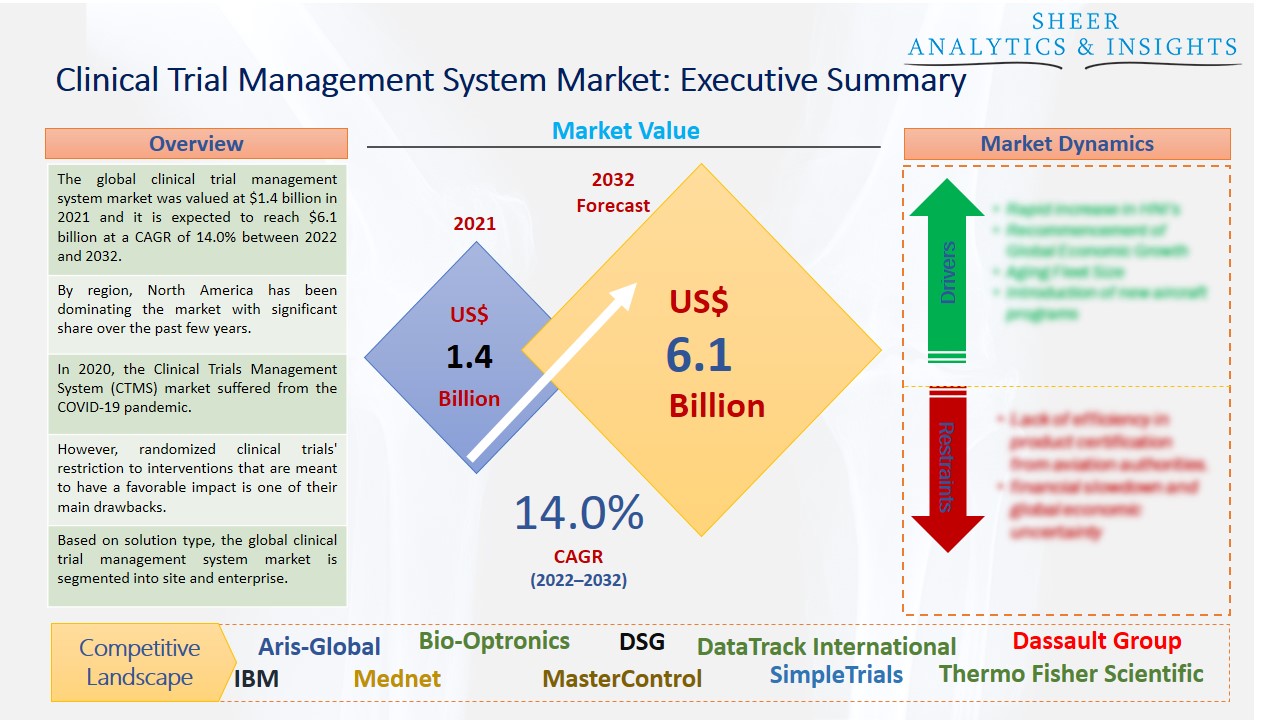
The global clinical trial management system market was valued at $1.4 billion in 2021 and it is expected to reach $6.1 billion at a CAGR of 14.0% between 2022 and 2032. The market is expected to increase as a result of the rapidly expanding healthcare IT industry, demand for decentralized clinical trials, initiatives by important companies, and an increase in the number of clinical studies.
By region, North America has been dominating the market with significant share over the past few years.
A cloud-based, highly configurable, end-to-end clinical trial management system called Clinical Trial Management System (CTMS) helps handle all elements of clinical trials, including Visits for Site Monitoring. Planning and tracking milestones Tasks, Activities, and Deviations. A software system called a clinical trial management system (CTMS) is used to handle clinical trials in clinical research. This CTMS will assist clinical research investigations carried out either within or across the three institutions as a unified, consolidated, web-based enterprise resource. The study startup, screening and enrollment, document collecting, site visits, monitoring reports, subject visit completion, action items, and issue management, among other crucial study management tasks, are all transparently supervised by the CTMS. A software system called a clinical trial management system (CTMS) is used to handle clinical trials in clinical research. This CTMS will assist clinical research investigations carried out either within or across the three institutions as a unified, consolidated, web-based enterprise resource. Due to these primary factors and benefits, the market is expected to have growth opportunities over the upcoming years.
Source: SAI Research
Download Free PDF Sample Request
In 2020, the Clinical Trials Management System (CTMS) market suffered from the COVID-19 pandemic. This included problems with clinical trials, difficulties finding patients, and postponed or canceled investigations. To assure the continuation of R&D, governments, regulatory organizations, and market stakeholders have adopted several strategic actions, which have steadily lessened the detrimental impact. Moreover, medical research efforts are being promoted by increased government financing as well as investments made by biotechnology and pharmaceutical companies. It is projected that this factor, together with technical improvements, will accelerate market expansion throughout the projection period. For instance, cloud-based CTMS removes the costs of purchasing, installing, deploying, maintaining, providing support, and licensing software.
However, randomized clinical trials' restriction to interventions that are meant to have a favorable impact is one of their main drawbacks. Because the examined population is so dissimilar from the population that is typically treated, it might be challenging to understand or generalize the results. The expensive clinical trial management system could potentially restrain the market's expansion. The combined cost for adopting CTMS solutions is increased by procurement, installation, and maintenance. About the number of users and sites taken into account, several pricing structures have been noticed. Along with routine system maintenance and upgrades, there are also certain contract or commitment term fees that must be taken into account for CTMS.
Based on solution type, the global clinical trial management system market is segmented into site and enterprise. The enterprise segment held the largest share of the market and is anticipated to accelerate the market growth during the forecast period. The main elements influencing this share are the accompanying advantages, including end-to-end insights into operational operations like accruals and variances, the scalability of the solution, robust reporting, improved billing compliance, and tracking and administration of regulatory processes. Due to developments in healthcare technology and organizational efforts to promote the healthcare sector, this trend is anticipated to continue during the projection period.
In terms of component type, the market is categorized into software, and services. The software sector represented the largest component share of the global Clinical Trials Management System (CTMS) market. Additionally, this segment is also expected to dominate the market during the forecast period from 2022 to 2032. The software assists in carrying out crucial tasks such as thorough trial planning, monitoring operations, regulatory processes, supply management, and financial management. These are often installed at the corporate or site level via subscription. Various biotechnology, pharmaceutical companies, and other life science organizations promoting research constantly conduct complex clinical trials. The goal of these studies is to find new medications and medical equipment. Furthermore, the sponsors can effectively manage clinical trials by using software-based technologies.
By delivery mode type, the web-based segment is estimated to boost the market growth in the future due to rising demand for web-based CTMS and growth in the number of clinical trials, the web-based segment, which was the largest contributor in 2020, is anticipated to keep up its dominance during the projection period. The web-based is quite effective because it can be used for many different study designs and has much functionality. The advantages of a web-based clinical trial management system (CTMS) include improved clinical trial quality and efficiency, better time management, and ease of access. On the other hand, the cloud-based segment is also anticipated to accelerate market growth throughout the forecast period. In addition, due to technological developments in the healthcare industry and a surge in the use of cloud-based systems, the cloud-based segment is anticipated to experience significant expansion during the projected period.
Based on end-user type, the large pharmaceutical biotech company category is projected to propel the market due to the growth of pharmaceutical and biopharmaceutical firms, as well as efforts made by both public and private entities to advance the pharmaceutical industry. By enhancing workflows, cutting expenses, and accelerating the entire clinical trial process, CTMS plays a crucial role in drug discovery. The CTMS market's greatest revenue share belongs to the category of pharmaceutical and biotech companies. The increasing use of CTMS by end users to store data, sync records, and handle the numerous clinical studies carried out each year is responsible for this growth.
Geographically, North America has been dominating the global clinical trial management system market over the past few years. Most of the key players are located in several countries in this region, which is an advantage for the various end-user industries. The presence of important enterprises and the growing use of technology in R&D can be linked to the region's considerable position in North America. This regional expansion might also be attributable to favorable regulatory policies and rising pharmaceutical company investment. One of the main reasons for the area's dominance in the worldwide CTMS market is the expanding clinical trial activity in North America, which is also accompanied by a higher cost of clinical operations in the region when compared to nations in the Asia Pacific and Latin America. On the other hand, the Asia-Pacific region also accounted for the largest share of the market due to increasing investment of government and key players in R&D activities. Private organizations are also supporting the market in making further growth opportunities.
According to the study, key players such as Aris-Global (U.S), Bio-Optronics (U.S), DSG (U.S), DataTrack International (U.S), Dassault Group (France), ENV (India), ERT (U.S), IBM (U.S), Mednet (U.S), MasterControl (U.S), Paraxel (U.S), SimpleTrials (U.S), Thermo Fisher Scientific (U.S), Oracle Corporation (U.S), Veeva Systems (U.S), Wipro (India), among others are leading the global clinical trial management system market.
Scope of the Report:
| Report Coverage | Details |
| Market Size in 2021 | US$ 1.4 Billion |
| Market Volume Projection by 2032 | US$ 6.1 Billion |
| Forecast Period 2022 to 2032 CAGR | 14.0% |
| Base Year: | 2021 |
| Historical Data | 2019, 2020 and 2021 |
| Forecast Period | 2022 to 2032 |
| Segments covered |
By Solution Type: Site, Enterprise By Component Type: Software, Services By Delivery Mode Type: Web-based CTMS, On-Premise, Cloud-based By End-User Type: Large Pharma Bio-tech Companies, Small and Mid-sized Pharma Bio-tech Companies, CROs, Others |
| Geographies covered |
North America, Europe, Asia-Pacific, LAMEA |
| Companies covered | Aris-Global (U.S), Bio-Optronics (U.S), DSG (U.S), DataTrack International (U.S), Dassault Group (France), ENV (India), ERT (U.S), IBM (U.S), Mednet (U.S), MasterControl (U.S), Paraxel (U.S), SimpleTrials (U.S), Thermo Fisher Scientific (U.S), Oracle Corporation (U.S), Veeva Systems (U.S), Wipro (India) & Others |
The Global Clinical Trial Management System Market Has Been Segmented Into:
The Global Clinical Trial Management System Market – by Solution Type:
- Site
- Enterprise
The Global Clinical Trial Management System Market – by Component Type:
- Software
- Services
The Global Clinical Trial Management System Market – by Delivery Mode Type:
- Web-based CTMS
- On-Premise
- Cloud-based
The Global Clinical Trial Management System Market – by End-User Type:
- Large Pharma Bio-tech Companies
- Small and Mid-sized Pharma Bio-tech Companies
- CROs
- Others
The Global Clinical Trial Management System Market – by Regions:
- North America
- The U.S.
- Canada
- Mexico
- Europe
- U.K.
- France
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- India
- China
- Japan
- Australia
- Rest of Asia Pacific
- LAMEA
- Middle East
- Saudi Arabia
- UAE
- Others
- Latin America
- Brazil
- Chile
- Others
- Africa
- South Africa
- Egypt
- Others
Buy Chapters or Sections
Customization options available to meet your custom research requirements :
- Request a part of this report
- Get geography specific report
- Request historical analysis
- Check out special discounted pricing

